
Comprehensive Analysis of O-linked Glycosylation
O-glycan Characterization using ImpaRATOR™
ImpaRATOR is an O-glycan-dependent protease that digests mucin-type
O-glycoproteins, including sialylated species, N-terminally of O-glycosylated serine and threonine residues.
This generates glycopeptides carrying O-glycans and enables detection of protein
O-glycosylation, O-glycan profiling and O-glycopeptide mapping using mass spectrometry. O-glycans are required for ImpaRATOR activity and the enzyme does not digest at
N-glycosylation sites of glycoproteins. The enzyme accepts a wide range of O-glycan species including sialylated core 1 and core 2 structures as well as the Tn antigen.
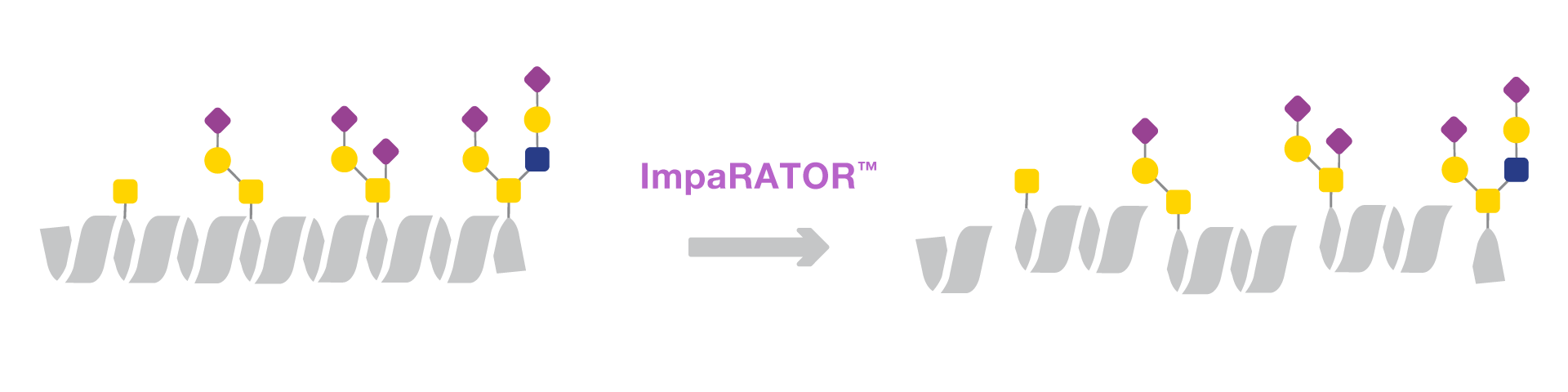



ImpaRATOR™ Accepts a Wide Range of O-glycan Species
Etanercept is a fusion protein consisting of the extracellular domain of the TNF receptor linked to the Fc portion of human IgG1. The protein contains a cluster of up to 13 O-glycosylated Ser/Thr residues just above the Fc region, making it a very difficult biopharmaceutical to characterize structurally. To demonstrate the broad glycan specificity of ImpaRATOR, three samples of etanercept with different glycan compositions were prepared: (i) the untreated glycoprotein carrying a mixture of mono- and di-sialylated core 1 structures, (ii) the glycoprotein treated with SialEXO to remove sialic acids and generate naked core 1 structures, and (iii) the glycoprotein treated with SialEXO and GalactEXO to generate single GalNAc residues, also known as Tn antigen. The three samples were treated with ImpaRATOR and FabRICATOR in a one-pot reaction (Fig. 1). As a comparison, SialEXO-treated etanercept was treated with OpeRATOR and FabRICATOR. The resulting peptides were reduced, alkylated and analyzed by HILIC-MS. FabRICATOR was included in the reaction to separate the Fc region from the most C-terminal glycopeptide to facilitate its characterization.
Figure 1. Schematic overview of the digestion workflow using ImpaRATOR.
Analysis of the ImpaRATOR-treated samples showed that ImpaRATOR digested all three substrates well and generated similar glycopeptides, but with the expected different glycan species (Fig. 2). The sample treated only with ImpaRATOR contained more variants than the other samples since each peptide was detected with several glycan structures. The observed glycan species were mainly mono- and di-sialylated core 1 structures, which is in accordance with the expected ones for etanercept. The SialEXO-pretreated samples digested with ImpaRATOR or OpeRATOR both resulted in peptides with naked core 1 structures, with the exception of peptide T205-P207 carrying the Tn antigen observed in the ImpaRATOR-digested sample. Etanercept pretreated with SialEXO and GalactEXO followed by digestion with ImpaRATOR resulted in peptides with only the Tn antigen. Together, this data demonstrates the high glycan substrate acceptance of ImpaRATOR. The ImpaRATOR-treated etanercept displayed a slightly different digestion pattern than the corresponding desialylated OpeRATOR-treated sample. For example, OpeRATOR digested between two O-glycosylated amino acids more efficiently than ImpaRATOR (for details, see section below). Etanercept with intact sialic acids or the Tn antigen was only digested very weakly by OpeRATOR (data not shown).
Figure 2. ImpaRATOR activity on etanercept with different glycan compositions. Etanercept a) untreated, b) treated with SialEXO, or c) treated with SialEXO and GalactEXO was digested by ImpaRATOR and FabRICATOR. d) Etanercept pretreated with SialEXO was digested with OpeRATOR and FabRICATOR. Peptides were reduced, alkylated and separated by HILIC (ACQUITY UPLC Glycoprotein Amide column, 300 Å, 1.7 μm, 2.1 mm × 150 mm) and analyzed on a Waters™ BioAccord™ LC-MS system. Shaded peaks indicate O-glycopeptides shared in (b) and (d).


ImpaRATOR™ and OpeRATOR® - Two Synergistic
O-glycoproteases
The coverage of O-glycan sites in the heavily glycosylated region of etanercept was analyzed after digestion using ImpaRATOR or OpeRATOR (Fig. 3). Peptides corresponding to all 13 O-glycan sites were identified using OpeRATOR, whereas only 10 sites could be unambiguously identified using ImpaRATOR. ImpaRATOR may have a more selective amino acid sequence preference than OpeRATOR (1), here indicated by the lack of digestion at sites S186 and T245. In addition, ImpaRATOR showed limited cleavage between two adjacent O-glycosylated amino acids, as demonstrated by the lack of peptides from digestion at site S213 and the low intensity of peptides from digestion at sites T200 and T217. The peptides from digestion at site T200 were of higher intensity in the OpeRATOR-digested sample (T184-S199 and T200-H204; Fig. 2b and d), whereas the corresponding peptide with a missed cleavage (S184-H204) was of a higher intensity in the ImpaRATOR-digested sample.
While OpeRATOR showed a higher O-glycan site coverage, digestion with ImpaRATOR enabled characterization of the O-glycan structures, including sialylated species. As an example, the bar graph inserts in Figure 3 show the O-glycan species for the two glycopeptides T184-V198 and T205-P207. This should be compared to digestion with OpeRATOR where the sialic acids had to be removed for enzyme activity, which means that the information about the sialylation is lost. To summarize, the ImpaRATOR and OpeRATOR enzymes together provide complete information about both the glycan composition and O-glycan sites.
Figure 3. Sequence coverage of etanercept after digestion with ImpaRATOR or OpeRATOR. Samples were prepared and data acquired as described in Figure 2. Data was processed in UNIFI (Waters™) using a Peptide Map (Exact Mass MS) method. Graph inserts show the glycan distribution of the two example peptides T184-V198 and T205-P207 after digestion with ImpaRATOR. Sites cleaved only by OpeRATOR are indicated by asterisks.
(1) Riley and Bertozzi, 2022. Deciphering O-glycoprotease substrate preferences with O-Pair. Mol. Omics., Dec 5;18(10):908-922.





