Efficient Hydrolysis of Galactose Using GalactEXO™
β-galactosidase for Efficient Hydrolysis of Galactose
GalactEXO is a mix of β-galactosidases for removal of galactose residues on N- and O-glycosylated proteins. The enzymes were discovered in Akkermansia muciniphila and characterization showed they efficiently hydrolyze β1-3 and β1-4 linked galactose. The combined galactosidase activities result in complete hydrolysis of galactose residues on 2 mg of native glycoprotein. GalactEXO can be used for trimming of released glycans in exoglycosidase array sequencing experiments where all galactose are removed within 1 h of incubation. The GalactEXO galactosidase mix can also be used to obtain a homogenous pool of therapeutic antibodies without galactose residues, G0 antibodies.

Schematic overview of the GalactEXO activity.
- Galactosidase hydrolyzing galactose residues on N- and O-glycoproteins
- β1-3 and β1-4 linked galactose
- 2 h incubation
- No co-factors required


Hydrolysis of β1-4 galactose on N-glycans
The β1-4 galactosidase activity of GalactEXO was demonstrated on trastuzumab that carries galactose residues in the Fc domain of the antibody. After 2 h of incubation with GalactEXO or competing products from other sources, the antibodies were digested into subunits using FabRICATOR® and analyzed using LC-MS (Fig. 1). The shift to G0F glycoforms can be seen using GalactEXO whereas enzymes from other sources results in incomplete hydrolysis.


Figure 1. Deconvoluted mass spectra of the Fc/2 fragment of trastuzumab treated with three different β-galactosidases. The mAb was digested with FabRICATOR and separated by reverse phase HPLC (Waters BioResolve RP, 2.1x100 mm) and analyzed by ESI-Q-TOF mass spectrometery (Bruker Impact II).


Galactosidase Activity on β1-3 Galactose from Etanercept
GalactEXO also display enzymatic activity on β1-3 linked galactose present on O-glycosylated biopharmaceuticals such as etanercept. Etanercept was incubated with GalactEXO or galactosidases from other sources for 2 h and the TNFR fragment was analyzed using LC-MS after FabRICATOR digestion to remove the Fc fragment (Fig. 2). The results show efficient galactosidase activity from GalactEXO compared to enzymes from other sources.
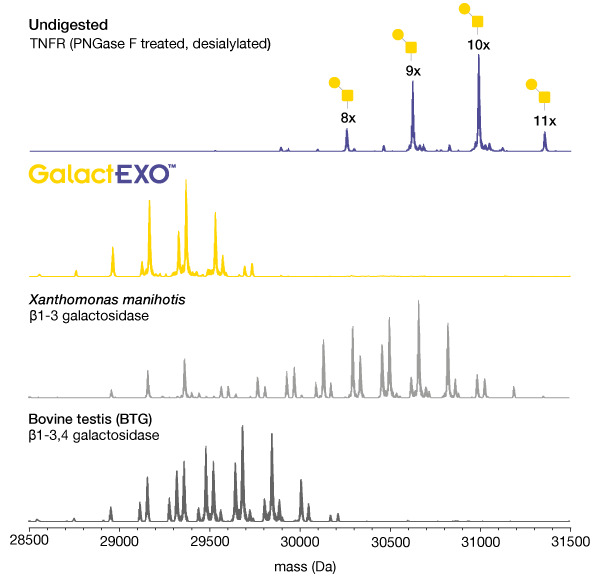

Figure 2. Deconvoluted mass spectra of the TNFR fragment of etanercept treated with three different β-galactosidases. The protein was digested with FabRICATOR, separated by reverse phase HPLC (Waters BioResolve RP, 2.1x100 mm) and analyzed by ESI-Q-TOF mass spectrometry (Bruker Impact II).


GalactEXO for Released Glycan Trimming
When analyzing released glycan structures using exoglycosidases it is crucial to obtain complete hydrolysis to minimize errors in data interpretation. A labelled N-glycan library was incubated with the sialidase SialEXO® and the galactosidase GalactEXO for 1 h and analyzed for the exoglycosidase activities (Fig. 3). The resulting HILIC separations show complete hydrolysis of both sialylated and galactosylated structures and the remaining peaks are easily identified as G0, G0F and G0F with bisecting GlcNAc.




Figure 3. HILIC-FLD HPLC chromatograms of a 2-AB labelled glycan library that was analyzed undigested (top) or after treatment with either SialEXO (middle) or the combination of SialEXO and GalactEXO (bottom). Analysis was performed on a Thermo Scientific Vanquish Duo UHPLC system equipped with a Thermo Scientific Accucore Amide HILIC column (2.1 x 150 mm).


Generating Antibodies with G0F Glycoforms
The N-glycans on IgG is crucial for the interactions with Fc receptors and are for this reason important for the development of therapeutic antibodies. GalactEXO can be used to generate antibodies with uniform glycosylation. By removing the terminal galactose using GalactEXO the remaining main glycoforms on the antibody is G0F with minor traces of G0 and Man5 glycans. The enzymatic reaction is complete within 2 h and the antibody could be used for functional studies such as mode of action analysis etc.


Figure 4: Degalactosylation of a mAb. Trastuzumab was treated with GalactEXO for 2h and the Fc glycosylation profile of the resulting mAbs was analyzed by middle-up LC-MS (top) as well as HILIC-FLD UHPLC analysis of released and fluorescently labelled glycans. Galactosylated glycoforms are shaded in yellow and are completely absent from the GalactEXO treated samples.